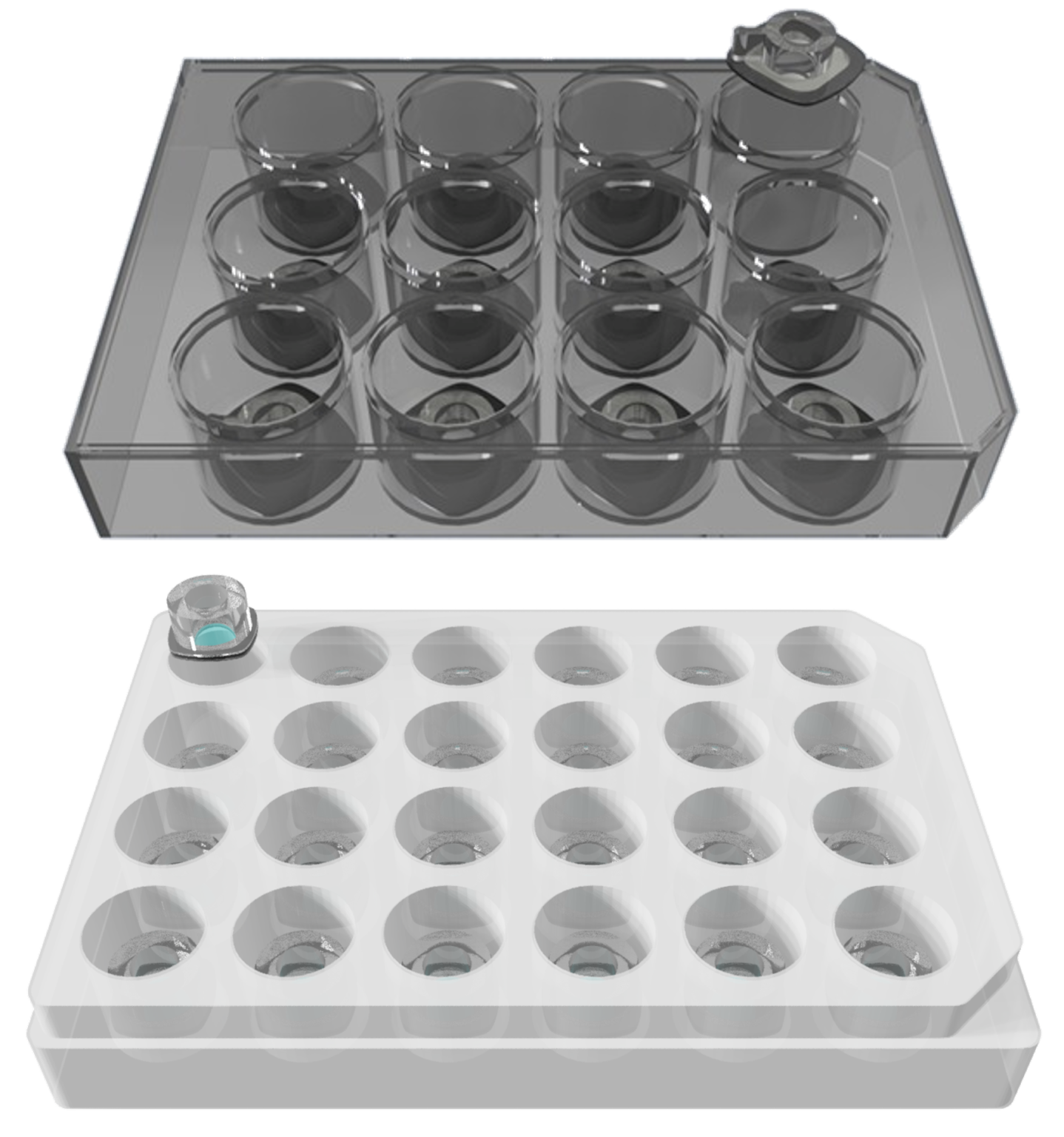
TTOP culture modules are designed to seamlessly integrate with 12 or 24 well plates, enabling conventional bicompartmental cultures without introducing technical complexity. Their standard design allows for a smooth transition from traditional insert-based models, making advanced tissue modeling accessible to a broader research community. The versatility of TTOP modules supports a wide variety of tissue samples, including cell monolayers, organoids, organotypic tissues, and patient-derived materials, all while allowing direct access and conventional handling.
These modules are fully compatible with standard Trans Epithelial
Electrical Resistance (TEER) measurement devices and permeability assays. Specifically designed for tissue barrier models, such as the gut, blood-brain barrier, cornea, and vascular systems, TTOP culture modules are set to impact biological and biomedical research by democratizing the use of microphysiological systems.

The TTOP TEER module is designed to seamlessly integrate with 12 or 24 culture modules, enabling the monitoring of TEER without requiring manual operations under a laminar flow hood. The TEER module canbe situated within an incubator and interfaced with EVOM electronics, allowing for programmable TEER measurements.
This setup eliminates the need for manual handling and minimizes potential positioning and temperature artifacts. Thanks to our proprietary biocompatible electrodes, the TEER module can remain in close proximity to cells without affecting their growth. This allows for the capture of rapid cell response dynamics to treatments, providing an unprecedented quantitative description of drug effects.
The control app enables researchers to set the total experimental period, select time points, and determine the number of technical replicates to measure at each time point, allowing them to program and automate the typically time-consuming, user-dependent, and cell-damaging TEER process.
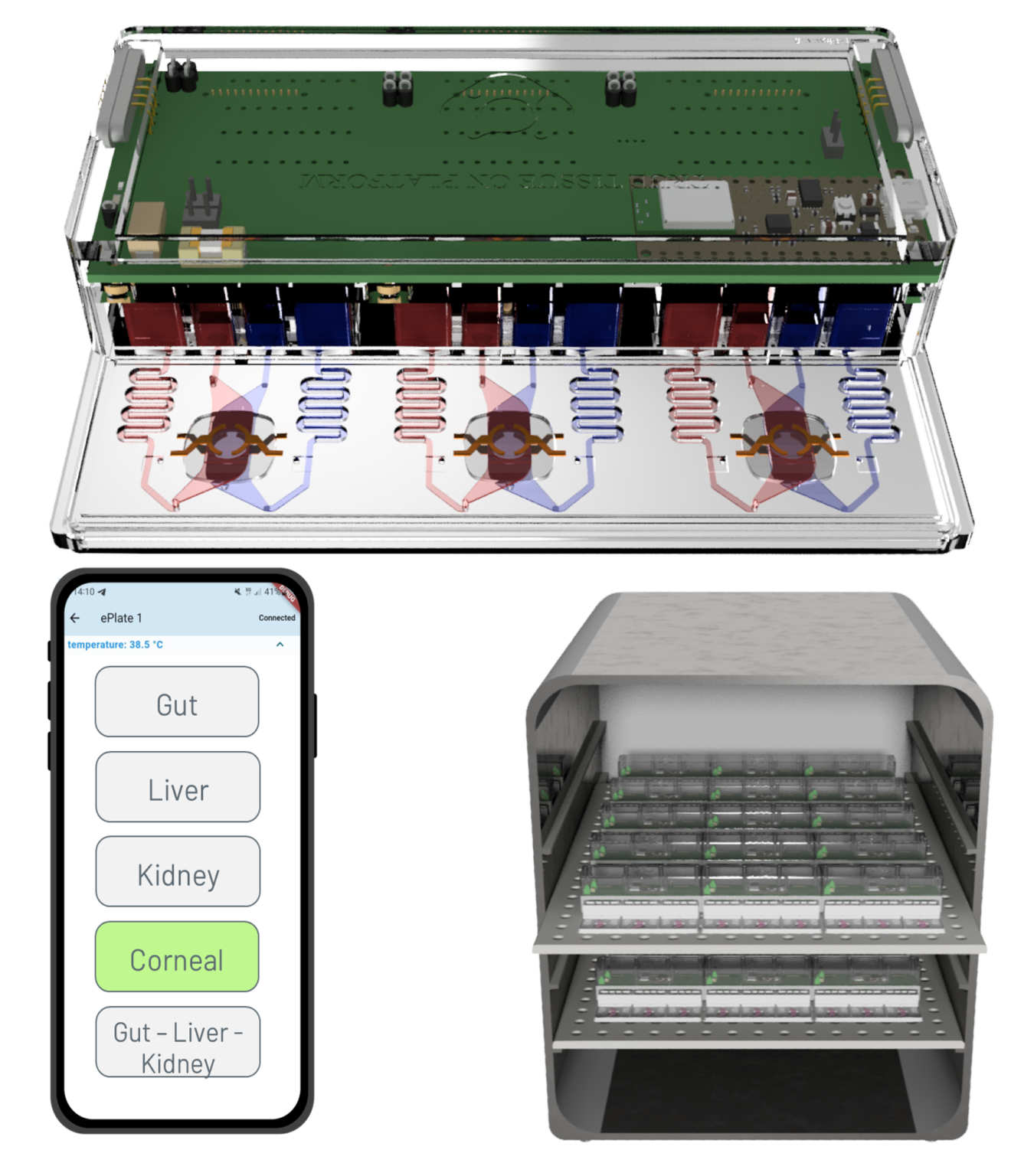
The TTOP perfusion modules are designed to host TTOP culture models with ease, allowing a seamless transition from static to perfused conditions for up to three replicates in a microtiter plate format. These modules reproduce controlled apical and basolateral physiological fluid flows, with integrated micropumps, eliminating the need for bulky accessories and tubing. The built-in TEER biosensors enable real-time, mini-invasive monitoring of barrier tissue functions, fully automating the process.
The compact and automated design facilitates scaling up the number of parallel devices without compromising result quality. Researchers can use the control app to independently set flow rates for each channel, offering a high degree of customization while automating the experimental process. Additionally, the plug-and-play design of the perfusion module allows for the connection of different models in a multi-organ configuration, enhancing experimental flexibility and complexity.